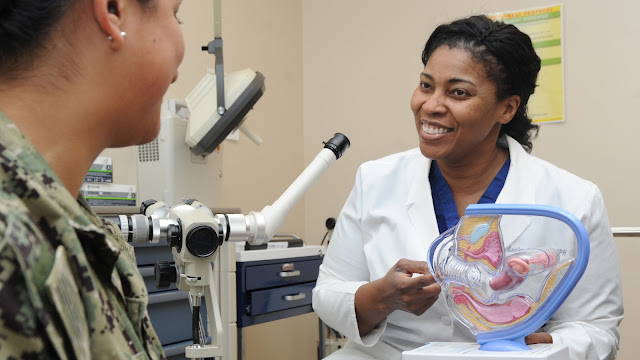
USU to Represent DoD in New Cancer Screening Research Network
The National Cancer Institute (NCI), part of the National Institutes of Health, has launched a new clinical trials network to evaluate emerging technologies for cancer screening in collaboration with the Uniformed Services University through its Murtha Cancer Center Research Program.
February 23, 2024 by Sarah Marshall
To identify cancers earlier and better understand when they may be easier to treat, the National Cancer Institute (NCI), part of the National Institutes of Health, has launched a new clinical trials network to evaluate emerging technologies for cancer screening. The Cancer Screening Research Network (CSRN), in support of the Biden-Harris Administration’s Cancer Moonshot, is a collaborative effort involving experts from institutions across the nation, including the Department of Defense’s Uniformed Services University (USU) through its Murtha Cancer Center Research Program (MCCRP).
CSRN will conduct rigorous multicenter cancer screening trials and studies with large and diverse populations in a variety of health care settings. Data collected through these clinical trials can be used to develop evidence-based guidelines for cancer screening.
“Murtha Cancer Center Research Program at USU is the hub for the federal Biden Cancer Moonshot activities within DoD and we are proud to be utilizing our extensive cancer research network at the eight largest DoD hospitals and medical centers to be able to enroll military, veteran, and beneficiary populations in these important cancer research studies,” said Dr. Craig Shriver, director of the Murtha Cancer Center (MCC)/Research Program (MCCRP).
As part of this effort, USU will represent the DoD on the Cancer Moonshot’s study of cancer detection in blood tests, which the Murtha Cancer Center plans to open later this year at all eight of its Murtha Research Network DoD hospital sites.
 |
| (Courtesy of NCI) |
The CSRN will also conduct rigorous, multi-center cancer screening trials and studies with large and diverse populations in a variety of health care settings with the ultimate goal of reducing cancer incidence and cancer-related morbidity and mortality. Cancer screening provides a unique opportunity to diagnose cancers and pre-cancerous lesions before symptoms develop.
As an initial effort, the CSRN will also launch a feasibility study, called the Vanguard study, to inform the future design of a platform trial to evaluate multiple different technologies for cancer screening in a flexible but rigorous manner. In 2024, NCI will begin enrolling about 24,000 healthy people aged 45-70 in the Vanguard study to assess the feasibility and finalize the design and logistics for a larger study. The Vanguard study will pave the way for future large-scale clinical trials and other studies developed to assess a variety of cancer screening modalities. The CSRN will use the NCI Clinical Trials Infrastructure, which includes a variety of integrated electronic systems, applications, and processes, which together facilitate the conduct of cancer clinical trials.
In addition, seven funded Accrual, Enrollment, and Screening Site (ACCESS) Hubs will lead efforts to enroll participants in their geographic and coverage areas. These ACCESS Hub include the Henry Ford Health Systems in Detroit and Michigan State University in East Lansing, Michigan; Kaiser Foundation Research Institute in Oakland, California; the University of Colorado in Aurora, Colorado; the University of North Carolina-Chapel Hill; the University of Oklahoma Health Sciences Center in Oklahoma City; the Virginia Commonwealth University in Richmond, Virginia; and the Washington University in Saint Louis.
The DoD, represented by USU/MCCRP, and the Veteran’s Health Systems will also participate as ACCESS Hubs funded by their respective agencies.
“NCI has launched CSRN to evaluate a variety of different technologies for the purpose of cancer screening,” said Dr. Lori M. Minasian, deputy director of NCI’s Division of Cancer Prevention. “Detecting cancer early is not enough to improve people’s lives. Through CSRN, we're going to study whether using these new technologies will make a difference in people’s lives.”